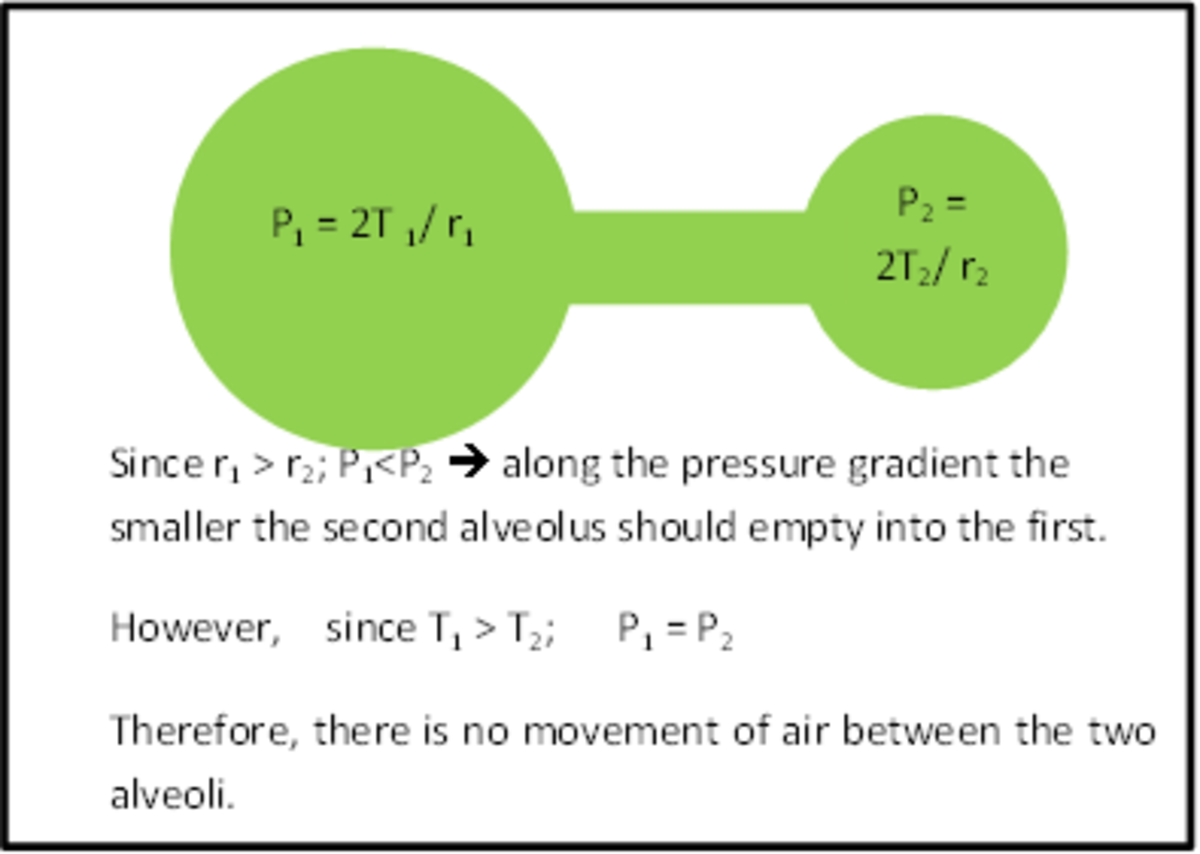
Why Reduce Surface Tension . surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. why does surface tension decrease with increasing temperature? surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. Increasing the temperature increases the kinetic.
from owlcation.com
this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. Increasing the temperature increases the kinetic. why does surface tension decrease with increasing temperature? surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air.
Surfactant Lowering Pulmonary Surface Tension Owlcation
Why Reduce Surface Tension this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. why does surface tension decrease with increasing temperature? Increasing the temperature increases the kinetic. water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Why Reduce Surface Tension this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. Increasing the temperature increases the kinetic. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. why does surface tension decrease with increasing temperature?. Why Reduce Surface Tension.
From www.kibron.com
Surface Tension What is surface tension Kibron Why Reduce Surface Tension why does surface tension decrease with increasing temperature? water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that. Why Reduce Surface Tension.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog Why Reduce Surface Tension surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension. Why Reduce Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Reduce Surface Tension this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in. Why Reduce Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube Why Reduce Surface Tension why does surface tension decrease with increasing temperature? surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension is a crucial property of a liquid that allows it to resist being pulled. Why Reduce Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Why Reduce Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. why does surface tension decrease with increasing temperature? this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. Increasing the temperature increases the kinetic.. Why Reduce Surface Tension.
From www.youtube.com
Why is surface tension important? YouTube Why Reduce Surface Tension Increasing the temperature increases the kinetic. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. water molecules at the surface of a drop or pool. Why Reduce Surface Tension.
From thechemistrynotes.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Why Reduce Surface Tension this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. Increasing the temperature increases the kinetic. water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. surface tension in water might be good at performing. Why Reduce Surface Tension.
From hubpages.com
Surfactant Lowering Pulmonary Surface Tension Owlcation Why Reduce Surface Tension this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Increasing the temperature increases the kinetic. why does surface tension decrease with increasing temperature?. Why Reduce Surface Tension.
From www.instructables.com
Surface TensionThrough Expirements 4 Steps Instructables Why Reduce Surface Tension surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. why does surface tension decrease with increasing temperature? Increasing the temperature increases the kinetic. surface tension in water might be good at performing tricks, such as being able to float a. Why Reduce Surface Tension.
From www.researchgate.net
Surface tension decreases with increasing the surfactant concentration Why Reduce Surface Tension water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. surface tension is a crucial property of a liquid that allows it to resist being pulled apart. Why Reduce Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Why Reduce Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Increasing the temperature increases the kinetic. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. water molecules at the surface of a drop. Why Reduce Surface Tension.
From www.muhadharaty.com
PulmonaryFunction pptx dr maha Muhadharaty Why Reduce Surface Tension surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. Increasing the temperature increases the kinetic. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules,. Why Reduce Surface Tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Why Reduce Surface Tension surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. why does surface tension decrease with increasing temperature? surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension is a crucial property of. Why Reduce Surface Tension.
From www.youtube.com
Surfactant and Surface Tension in Respiration Breathing Mechanics Why Reduce Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Increasing the temperature increases the kinetic. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower.. Why Reduce Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Why Reduce Surface Tension Increasing the temperature increases the kinetic. surface tension is a fundamental concept in fluid dynamics, playing a crucial role in phenomena. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. why does surface tension decrease with increasing temperature? surface. Why Reduce Surface Tension.
From www.vrogue.co
Surfactant Lowering Pulmonary Surface Tension Owlcati vrogue.co Why Reduce Surface Tension why does surface tension decrease with increasing temperature? water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. this surface energy per unit area can be alternatively described as “interfacial tension” (ift), which is a. Increasing the temperature increases the kinetic. . Why Reduce Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Reduce Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower. why does surface tension decrease. Why Reduce Surface Tension.